Chemistry Notes
Structure of Ethene
Ethene is not a very complicated molecule. It contains two carbon atoms that are double bonded to each other, with each of these atoms also bonded to two Hydrogen atoms.

This forms a total of three bonds to each carbon atom, giving them an hybridization. Since the carbon atom is forming three sigma bonds instead of the four that it can, it only needs to hybridize three of its outer orbitals, instead of four. It does this by using the electron and two of the electrons, leaving the other unchanged. This new orbital is called an hybrid because that's exactly what it is, it is made from one s orbital and two p orbitals.
.png?revision=1)
When atoms are an hybrid they have a trigonal planar structure. These structures are very similar to a 'peace' sign, there is a central atom with three atoms around it, all on one plane. Trigonal planar molecules have an ideal bond angle of 120° on each side.
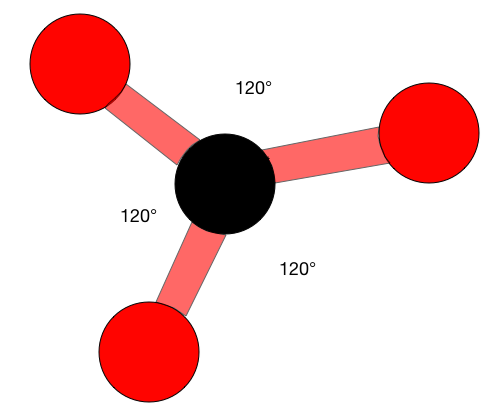
The H-C-H bond angle is 117°, which is very close to the ideal 120° of a carbon with hybridization. The other two angles (H-C=C) are both 121.5°.

Comments
Post a Comment