Chemistry Notes
Extraction of Aluminium
A soft, silvery-white, corrosion-resistant metal. It is the most abundant metal in the earth’s crust as it makes up 8% of the crust and it is the third most abundant element after oxygen and silicon. Bauxite ore (Al2O3.xH2O) is the major source of aluminium till date which is a mixture of hydrated aluminium oxide.
Aluminium can also be recovered from cryolite (Na3AlF6) and alunite. It is also found in gemstones such as garnet, topaz and chrysoberyl. The chemical symbol of this metal is Al. In the boron group with symbol Al, aluminium is a chemical element and is the most commonly used non-ferrous metal.
A soft, silvery-white, corrosion-resistant metal. It is the most abundant metal in the earth’s crust as it makes up 8% of the crust and it is the third most abundant element after oxygen and silicon. Bauxite ore (Al2O3.xH2O) is the major source of aluminium till date which is a mixture of hydrated aluminium oxide.
Aluminium can also be recovered from cryolite (Na3AlF6) and alunite. It is also found in gemstones such as garnet, topaz and chrysoberyl. The chemical symbol of this metal is Al. In the boron group with symbol Al, aluminium is a chemical element and is the most commonly used non-ferrous metal.
Aluminium Ore
Ores of Aluminium
Aluminium is a highly reactive metal, belonging to the IIIA group of the periodic table. In nature, aluminium is found in the form of its oxide in its ores. The important ores of aluminium are
- Bauxite – Al2O3.2H2O
- Corundum – Al2O3
- Cryolite – Na3AlF6
Aluminium is a highly reactive metal, belonging to the IIIA group of the periodic table. In nature, aluminium is found in the form of its oxide in its ores. The important ores of aluminium are
- Bauxite – Al2O3.2H2O
- Corundum – Al2O3
- Cryolite – Na3AlF6
Metallurgy of Aluminium
Aluminium is mostly extracted from its bauxite ore. Dressing of ore: The ore is crushed and pulverized.
Aluminium is mostly extracted from its bauxite ore. Dressing of ore: The ore is crushed and pulverized.
Concentration of ore
The bauxite ore contains ferric oxide and silica as impurities. It is first concentrated by gravity separation of ferric oxide impurities by the process of magnetic separation. The ore is then concentrated by chemical process.
Bauxite is the name given to aluminium ore. To generate aluminium oxide, bauxite is purified, a white powder form which aluminium can be extracted. Aluminium oxide has a very high melting point of more than 2000 ° C so melting it would be costly. Aluminium oxide in water does not dissolve, but in molten cryolite, it dissolves.

Pure aluminium is a silver-white metal with many desirable features. It’s light, non-toxic, non-magnetic, and non-sparking. It’s a bit ornamental. It’s created, machined, and cast readily. Pure aluminium is soft and lacks strength, but it has very helpful characteristics for alloys with tiny quantities of copper, magnesium, silicon, manganese and other components.
The bauxite ore contains ferric oxide and silica as impurities. It is first concentrated by gravity separation of ferric oxide impurities by the process of magnetic separation. The ore is then concentrated by chemical process.
Bauxite is the name given to aluminium ore. To generate aluminium oxide, bauxite is purified, a white powder form which aluminium can be extracted. Aluminium oxide has a very high melting point of more than 2000 ° C so melting it would be costly. Aluminium oxide in water does not dissolve, but in molten cryolite, it dissolves.

Pure aluminium is a silver-white metal with many desirable features. It’s light, non-toxic, non-magnetic, and non-sparking. It’s a bit ornamental. It’s created, machined, and cast readily. Pure aluminium is soft and lacks strength, but it has very helpful characteristics for alloys with tiny quantities of copper, magnesium, silicon, manganese and other components.
Bayer’s Process
In this process, aluminium ore is treated with concentrated sodium hydroxide. Soluble sodium aluminate is formed which is filtered off. The filtrate on heating with water gives aluminium hydroxide which gives alumina on strong heating.
In this process, aluminium ore is treated with concentrated sodium hydroxide. Soluble sodium aluminate is formed which is filtered off. The filtrate on heating with water gives aluminium hydroxide which gives alumina on strong heating.
Hall-Heroult Process
The Hall-Heroult process is widely used in the extraction of aluminium. In Hall-Heroults process, pure Al2O3 is mixed with CaF2 or Na3AlF6. This results in lowering of the melting point of the mixture and increases its ability to conduct electricity. A steel vessel with the lining of carbon and graphite rods is used.
The carbon lining acts as cathode and graphite act as an anode. When electricity is passed through the electrolytic cell which consists of carbon electrodes oxygen is formed at the anode. This oxygen formed reacts with the carbon of the anode to form carbon monoxide and carbon dioxide. In this method of production of aluminium for every 1 kg of Al produced, approximately 0.5 Kg of carbon anode is burnt.
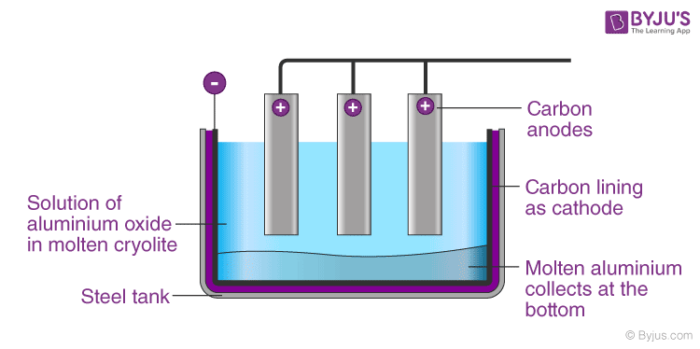
Aluminium ions are created at the adverse cathode from the aluminium oxide and then sink down because they are heavier than the cryolite solution. Then, the liquid shape of the aluminium that has sunk to the bottom. On the other side, at the positive anode, the oxygen from the aluminium oxide forms and responds to carbon dioxide CO2 with the graphite carbon.
The overall reaction is:
2Al2O3 + 3C → 4Al + 3CO2
The electrolytic reactions are:
The Hall-Heroult process is widely used in the extraction of aluminium. In Hall-Heroults process, pure Al2O3 is mixed with CaF2 or Na3AlF6. This results in lowering of the melting point of the mixture and increases its ability to conduct electricity. A steel vessel with the lining of carbon and graphite rods is used.
The carbon lining acts as cathode and graphite act as an anode. When electricity is passed through the electrolytic cell which consists of carbon electrodes oxygen is formed at the anode. This oxygen formed reacts with the carbon of the anode to form carbon monoxide and carbon dioxide. In this method of production of aluminium for every 1 kg of Al produced, approximately 0.5 Kg of carbon anode is burnt.

Aluminium ions are created at the adverse cathode from the aluminium oxide and then sink down because they are heavier than the cryolite solution. Then, the liquid shape of the aluminium that has sunk to the bottom. On the other side, at the positive anode, the oxygen from the aluminium oxide forms and responds to carbon dioxide CO2 with the graphite carbon.
The overall reaction is:
2Al2O3 + 3C → 4Al + 3CO2
The electrolytic reactions are:
At the cathode:
Al 3+ + 3e– → Al (l)
Al 3+ + 3e– → Al (l)
At the anode:
C (s) + O2- → CO (g) + 2e–
C (s) + 2O2- → CO2 (g) + 4e–
During the process of electrolysis,
- Aluminium ions that are positively loaded gain electrons from the cathode and form molten aluminium.
- Oxide ions lose anode electrons and form molecules of oxygen
In the electrochemical sequence means reactivity series, aluminium is too big to be removed from its ore by carbon reduction. The required temperatures are too high to be economical.
C (s) + O2- → CO (g) + 2e–
C (s) + 2O2- → CO2 (g) + 4e–
During the process of electrolysis,
- Aluminium ions that are positively loaded gain electrons from the cathode and form molten aluminium.
- Oxide ions lose anode electrons and form molecules of oxygen
In the electrochemical sequence means reactivity series, aluminium is too big to be removed from its ore by carbon reduction. The required temperatures are too high to be economical.

Comments
Post a Comment